Directive 2010/63/EU, the European Union’s framework for the protection of animals used in research, represents one of the strongest policy commitments worldwide to reduce, refine, and replace animal testing. Since coming into force in 2013, it has required every EU Member State to hold researchers accountable for proving that animal use is necessary — and to prioritize alternatives whenever they exist.
At the core of the Directive are the 3Rs:
- Replacement — use non-animal methods when possible,
- Reduction — minimize the number of animals involved,
- Refinement — improve welfare and reduce suffering in any remaining animal work.
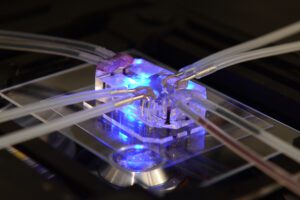
For the organoid field, this Directive is more than regulation — it is a signal of opportunity. By mandating that scientists justify why an animal must be used, the EU has created a powerful incentive to invest in human-based models. Organoids, with their ability to capture the genetic, molecular, and functional complexity of human tissues, are a direct response to this call.
Our work in automating organoid testing aligns seamlessly with the goals of Directive 2010/63/EU. Automation ensures that organoid systems can be scaled, standardized, and validated at the level regulators demand. By reducing variability and increasing throughput, automation makes organoids not just an ethical alternative but a reliable, practical one — capable of replacing animal studies where they fall short.
Europe’s leadership on this issue highlights a global shift: the future of biomedical research is human-relevant. Directives like 2010/63/EU don’t just protect animals — they accelerate innovation, efficiency, and scientific accuracy. And as the field advances, companies committed to building robust, automated organoid platforms will be at the forefront of that transformation.stitutions in Europe must comply with its rules or face legal and ethical consequences. EUR-Lex+1